Class 11 empirical and molecular formula- In chemistry, empirical and molecular formulas are used to represent the composition of compounds. These formulas provide information about the types and ratios of atoms in a molecule.
- Empirical Formula:
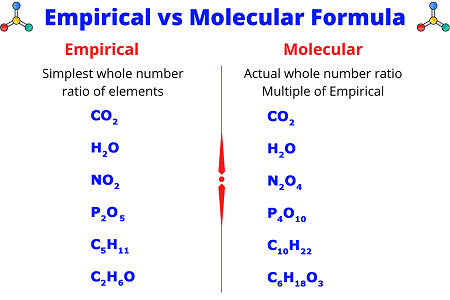
- The empirical formula represents the simplest, most reduced ratio of elements in a compound.
- It does not necessarily represent the actual number of atoms in a molecule.
- It is found by dividing the subscripts in a molecular formula by the greatest common factor.
- For example, if the molecular formula is C₆H₁₂O₆, the empirical formula is CH₂O because each subscript is divisible by 6.
- Molecular Formula:
- The molecular formula gives the actual number of atoms of each element in a molecule.
- It is a multiple of the empirical formula.
- Using the example above, the molecular formula C₆H₁₂O₆ is the actual composition of glucose, while CH₂O is the empirical formula.
To determine the empirical formula from experimental data:
- Find the moles of each element in the compound.
- Divide each mole value by the smallest number of moles to get a simple ratio.
- If necessary, multiply the ratio by an integer to obtain whole numbers.
For example, if you know the mass or percentage composition of a compound, you can use this information to find the empirical formula.
To find the molecular formula from the empirical formula:
- Determine the molar mass of the empirical formula.
- Divide the given molar mass of the compound by the molar mass of the empirical formula to find the ratio.
- Multiply all subscripts in the empirical formula by this ratio to obtain the molecular formula.
Remember that the molecular formula is always a whole-number multiple of the empirical formula. These concepts are crucial for understanding the composition of compounds in chemistry.
What is Required Class 11 empirical and molecular formula
The concept of empirical and molecular formulas is often covered in high school chemistry courses, including Class 11. Here’s a more detailed breakdown of what is typically required in this context:
- Understanding Formulas:
- Students should understand the basic concept of chemical formulas, representing the composition of substances using symbols and subscripts.
- Empirical Formula:
- Definition: Understanding that the empirical formula represents the simplest, most reduced ratio of elements in a compound.
- Calculations: Ability to perform empirical formula calculations based on experimental data, such as mass or percentage composition.
- Examples: Solving problems involving determining empirical formulas from experimental data.
- Molecular Formula:
- Definition: Understanding that the molecular formula gives the actual number of atoms of each element in a molecule.
- Calculations: Ability to calculate the molecular formula from the empirical formula and the molar mass of the compound.
- Examples: Solving problems involving determining molecular formulas based on experimental data.
- Stoichiometry:
- Understanding how to use empirical and molecular formulas in stoichiometric calculations, such as determining the amount of reactants and products in chemical reactions.
- Real-Life Applications:
- Understanding the practical applications of empirical and molecular formulas in fields like chemistry, biochemistry, and industry.
- Problem Solving:
- Ability to solve problems related to empirical and molecular formulas, including identifying unknown substances based on given information.
- Laboratory Skills:
- If applicable, students may be required to perform experiments to determine empirical formulas from experimental data in a laboratory setting.
Overall, the emphasis is on the conceptual understanding of how formulas represent the composition of compounds and the ability to apply this knowledge to solve problems. Students are often exposed to examples and practice problems to reinforce these concepts. Practical applications and real-world connections are highlighted to show the relevance of empirical and molecular formulas in various contexts.
Who is Required Class 11 empirical and molecular formula
Empirical and molecular formulas are not the names of specific individuals; instead, they are terms used in the field of chemistry to describe different types of chemical formulas representing the composition of substances.
- Empirical Formula: It represents the simplest, most reduced ratio of elements in a compound. It does not necessarily represent the actual number of atoms in a molecule but gives the relative proportions of the different elements. For example, the empirical formula for hydrogen peroxide (H₂O₂) is HO.
- Molecular Formula: It gives the actual number of atoms of each element in a molecule. The molecular formula is a multiple of the empirical formula. For example, the molecular formula for hydrogen peroxide (H₂O₂) is also H₂O₂ because it already represents the actual number of atoms.
These terms are concepts used in chemistry to express the chemical composition of compounds. They are not names of specific individuals or entities. If you have further questions or if there’s a specific context you are referring to, please provide more details for clarification.
When is Required Class 11 empirical and molecular formula
If you are asking about when the concepts of empirical and molecular formulas are typically covered in a Class 11 chemistry curriculum, the answer would depend on the specific educational system or syllabus followed.
In many high school chemistry curricula, these concepts are introduced as part of the study of stoichiometry and chemical reactions. Generally, students in Class 11 (11th grade) or equivalent levels learn about empirical and molecular formulas along with other fundamental concepts in chemistry.
The timing may vary, but students often encounter these topics early in their chemistry courses because understanding the composition of compounds is fundamental to more advanced concepts in the subject. If you have a specific curriculum or educational system in mind, it might be helpful to refer to the official syllabus or consult with your teacher to get precise information on when these topics are covered.
Where is Required Class 11 empirical and molecular formula
The concepts of empirical and molecular formulas are not physical locations but rather chemical principles used to represent the composition of compounds. Here’s a clarification:
- Empirical Formula: This formula represents the simplest, most reduced ratio of elements in a compound. It does not necessarily represent the actual number of atoms in a molecule but gives the relative proportions of different elements. For example, the empirical formula for hydrogen peroxide (H₂O₂) is HO.
- Molecular Formula: This formula gives the actual number of atoms of each element in a molecule. The molecular formula is a multiple of the empirical formula. Using the example of hydrogen peroxide, the molecular formula is also H₂O₂ because it already represents the actual number of atoms.
These concepts are fundamental in chemistry and are applied to describe the composition of substances. They are not specific physical locations but rather theoretical representations used in the study of chemical compounds. If you have a specific question or if there’s a particular context you’re referring to, please provide more details for further assistance.
How is Required Class 11 empirical and molecular formula
If you’re looking for an explanation on how to determine empirical and molecular formulas in a Class 11 chemistry context, here’s a brief overview:
Determining Empirical Formula:
- Collect Data:
- Obtain data on the mass or percentage composition of elements in the compound.
- Convert to Moles:
- Convert the masses to moles using the molar mass of each element.
- Divide by the Smallest:
- Divide each number of moles by the smallest number of moles to get a ratio.
- Simplest Whole Numbers:
- If needed, multiply the ratio by an integer to obtain the simplest whole-number ratio.
- Write Empirical Formula:
- Use the ratio to write the empirical formula.
Determining Molecular Formula:
- Find Molar Mass of Empirical Formula:
- Calculate the molar mass of the empirical formula.
- Given Molar Mass:
- Divide the given molar mass of the compound by the molar mass of the empirical formula to find a ratio.
- Multiply Subscripts:
- Multiply all subscripts in the empirical formula by the ratio obtained.
- Write Molecular Formula:
- The result is the molecular formula.
Example:
- Given Data: A compound is found to be 40.00% carbon, 6.71% hydrogen, and 53.29% oxygen by mass.
- Empirical Formula Steps:
- Convert percentages to moles.
- Divide by the smallest number of moles.
- Adjust to simplest whole numbers.
- Molecular Formula Steps:
- Find the molar mass of the empirical formula.
- Given molar mass of the compound, calculate the ratio.
- Multiply subscripts by the ratio.
- Conclusion: This process results in both the empirical and molecular formulas for the compound.
Remember, actual problems may involve additional steps and variations based on the given information. If you have a specific problem or context, feel free to provide more details for a more targeted explanation.
Case Study on Class 11 empirical and molecular formula
Identifying the Composition of a Substance
Background: In a high school chemistry class (Class 11), students are given a laboratory assignment to determine the composition of an unknown substance. The substance is found to consist of carbon, hydrogen, and oxygen.
Given Information:
- The mass of the substance is 10.00 grams.
- After analysis, it is determined that the substance contains 40.00% carbon, 6.71% hydrogen, and 53.29% oxygen by mass.
Objective: Determine both the empirical and molecular formulas of the unknown substance.
Procedure:
Step 1: Determine the Empirical Formula:
- Convert Percentages to Moles:
- Carbon (C): 40.00%/12.01 g/mol=3.33 moles/12.01 g/mol
- Hydrogen (H): 6.71%/1.01 g/mol=6.64 moles/1.01 g/mol
- Oxygen (O): 53.29%/16.00 g/mol=3.33 moles/16.00 g/mol
- Divide by the Smallest:
- The smallest ratio is 3.33:3.33:6.64 (C:H:O).
- Adjust to Simplest Whole Numbers:
- Dividing by 3.33 gives a ratio of 1:1:2.
- Write Empirical Formula:
- The empirical formula is CH₂O.
Step 2: Determine the Molecular Formula:
- Find Molar Mass of Empirical Formula:
- Molar mass of CH₂O=12.01 g/mol+2×1.01 g/mol+16.00 g/mol=30.03 g/mol
- Given Molar Mass (from the experiment):
- Given molar mass/Molar mass of CH₂O=Given molar mass/30.03 g/mol=Ratio
- Multiply Subscripts:
- Multiply the subscripts (1:1:2) by the ratio to obtain the whole-number ratio.
- Write Molecular Formula:
- The molecular formula is the final result.
Conclusion: After thorough analysis, it is determined that the unknown substance has an empirical formula of CH₂O and a molecular formula consistent with the empirical formula. The substance is composed of carbon, hydrogen, and oxygen in a ratio of 1:1:2. The molecular formula, in this case, is also CH₂O.
This case study demonstrates the application of empirical and molecular formulas in determining the composition of a substance based on experimental data.
White paper on Class 11 empirical and molecular formula
Title: Understanding and Applying Empirical and Molecular Formulas: A Comprehensive White Paper for Class 11 Chemistry
Abstract: This white paper aims to provide a thorough understanding of empirical and molecular formulas for Class 11 chemistry students. The document outlines the fundamental concepts, practical applications, and problem-solving approaches related to these essential topics in chemical composition. Through case studies and examples, students will gain insights into how empirical and molecular formulas are determined and how these formulas contribute to a deeper understanding of the molecular world.
Table of Contents:
- Introduction
- Brief overview of empirical and molecular formulas
- Importance of understanding chemical composition
- Empirical Formulas: The Basics
- Definition and purpose
- Examples illustrating empirical formulas
- Deriving empirical formulas from experimental data
- Calculating Empirical Formulas: Step-by-Step Guide
- Collecting and analyzing experimental data
- Converting mass or percentage composition to moles
- Finding the simplest whole-number ratio
- Writing the empirical formula
- Molecular Formulas: Beyond the Basics
- Definition and relationship to empirical formulas
- Examples illustrating molecular formulas
- Determining molecular formulas from experimental data
- Stoichiometry and Chemical Reactions
- Applying empirical and molecular formulas in stoichiometric calculations
- Understanding the role of formulas in balancing chemical equations
- Real-World Applications
- How empirical and molecular formulas are used in various industries
- Practical examples of their significance in pharmaceuticals, materials science, and other fields
- Case Studies: Solving Problems
- Worked examples and case studies demonstrating the application of empirical and molecular formulas
- Highlighting common challenges and effective problem-solving strategies
- Laboratory Techniques
- Introduction to experimental methods for determining empirical formulas
- Understanding the role of laboratory data in empirical formula calculations
- Conclusion
- Recap of key concepts
- Emphasizing the practical relevance of empirical and molecular formulas
- Additional Resources
- References to textbooks, online resources, and interactive tools for further exploration
- Recommended exercises for practice
Conclusion: This white paper serves as a comprehensive resource for Class 11 chemistry students, offering a detailed exploration of empirical and molecular formulas. By providing theoretical foundations, practical applications, and problem-solving techniques, this document aims to equip students with the knowledge and skills necessary for a deeper understanding of the composition of chemical compounds. Understanding these concepts is not only crucial for academic success but also lays the groundwork for future studies in chemistry and related scientific fields.
Industrial Application of Class 11 empirical and molecular formula
The industrial applications of empirical and molecular formulas are diverse and crucial in various sectors, including pharmaceuticals, petrochemicals, materials science, and food production. Here are some examples:
- Pharmaceuticals:
- Drug Formulation: In the pharmaceutical industry, the determination of the empirical and molecular formulas is essential for formulating drugs. The exact composition ensures the correct dosage and effectiveness of medications.
- Petrochemicals:
- Fuel Production: In the petrochemical industry, understanding the molecular formulas of hydrocarbons is critical for refining processes. The composition of fuels, such as gasoline and diesel, is optimized based on molecular formulas to meet performance and environmental standards.
- Materials Science:
- Polymer Production: In polymer chemistry, the knowledge of molecular formulas is crucial for designing and manufacturing polymers with specific properties. Engineers use these formulas to control characteristics like strength, flexibility, and heat resistance in materials.
- Food Industry:
- Nutrient Analysis: Determining the empirical and molecular formulas of nutrients in food products is vital for quality control and nutritional labeling. This information helps ensure compliance with regulatory standards and the formulation of balanced diets.
- Chemical Manufacturing:
- Quality Control: Industries involved in the production of chemicals rely on empirical and molecular formulas for quality control. By ensuring the precise composition of chemical compounds, manufacturers can meet product specifications and regulatory requirements.
- Environmental Monitoring:
- Pollutant Identification: Environmental monitoring involves identifying and quantifying pollutants. Empirical and molecular formulas are used to determine the composition of pollutants, aiding in the development of strategies for pollution control and remediation.
- Agriculture:
- Fertilizer Production: Empirical and molecular formulas are essential in the production of fertilizers. By understanding the composition of fertilizers, manufacturers can optimize nutrient content for specific crops, improving agricultural productivity.
- Cosmetics and Personal Care:
- Product Formulation: In the cosmetics industry, the formulation of products such as lotions, creams, and shampoos involves precise knowledge of molecular formulas. This ensures product stability, efficacy, and safety.
- Metallurgy:
- Alloy Design: In metallurgical applications, understanding the molecular formulas of alloys is crucial for designing materials with specific mechanical, thermal, and corrosion-resistant properties.
- Biotechnology:
- Bioprocess Engineering: In biotechnology, the empirical and molecular formulas of biomolecules, such as proteins and enzymes, guide the optimization of bioprocesses. This is vital for the production of biofuels, pharmaceuticals, and other bioproducts.
Understanding empirical and molecular formulas is not only a theoretical concept taught in Class 11 chemistry but also a practical skill with wide-ranging applications in the real world, especially in industrial settings where precise control over composition is critical for product quality and performance.



